Birds do it, bees do it—even laboratory mice do it. But with science in the mix, actually creating new life may not always require a male and a female.
Using gene editing and stem cells, researchers in China have helped mice of the same sex bear pups. While this feat has been accomplished before with mouse moms, the new study marks the first time that pups from pairs of male mice were also carried to full term.
The technology is far from ready for the leap to humans. Though mice pups born from two females appeared healthy and bore their own young, pups with two papas died soon after birth. Of the 12 born, just two survived more than 48 hours.
Still, the new study, published today in the journal Cell Stem Cell, is an encouraging step toward a better understanding of the barriers that prevent such genetic coupling between individuals of the same sex. The work also raises a slew of ethical questions among experts, with the health of future offspring being the primary concern.
“When you do the gene targeting, you may get some unintended side effects. You may alter other sequences which you didn't mean to alter,” says Azim Surani, a developmental biologist at the University of Cambridge who was not involved in the study. These changes in the genome are passed from one generation to the next, along with any potentially negative side effects.
At this point, the researchers aren't focused on translating the results to humans, but it's not an impossibility: “We can’t assert this technique could never be used in humans in the future,” senior author Wei Li from the Chinese Academy of Sciences says via email.
“We're going to have to really think hard, as a society, about what our threshold should be for doing this kind of research,” says Sonia Suter, a law professor at George Washington University who specializes in bioethics and health policy.
Forgoing fatherhood
The new study is one in a series of works attempting to get around an issue called imprinting. In humans, genes are packed into 23 pairs of chromosomes—you inherit one set from mom and another from dad. Yet many creatures don't develop in the same configuration. A select few vertebrates can have babies without genetic input from a male—some types of lizards, frogs, and even fish can bear young without fathers. Oftentimes, this so-called parthenogenesis is spurred by captivity.
But that's not the case for mammals with a placenta, the disc of tissue that helps facilitate the exchange of nutrients and waste between mother and baby.
“There's a barrier, and that barrier is imprinting,” Surani explains. Imprinting happens during the sperm and egg development, when “tags” attach to your chromosomes, influencing the gene's function. For some reason, the set of tags is different in chromosomes from each parent. Some genes need to be active in DNA from mom, others need to be active in DNA from dad.
It's not entirely known why this process happens in placental mammals, says Surani, who discovered the curious phenomenon in 1984. One general thought is that these tags help balance embryo development. But he emphasizes that scientists have floated many explanations.
“We just don't know,” he says.
Making a bimaternal mouse
For the latest work, the scientists relied on what's known as haploid embryonic stem cells. These cells have just a single set of chromosomes and are cultured from sperm or egg cells, reducing the number of problematic genetic tags.
The researchers then used the “molecular scissors” known as CRISPR-Cas9 to snip out segments known to prove troublesome for imprinting. For female mouse pairs, they had to delete three locations to get healthy young. For male mouse pairs, they had to snip seven regions. (Learn about the first edited human embryos.)
The next steps for female mice were relatively straightforward: The researchers transferred the altered stem cells into an unchanged immature egg, which at this stage looks like a tentacle-free jellyfish thanks to its protective gelatinous coating, senior study author Baoyang Hu says via email. The researchers then inserted the egg into a surrogate mouse for development.
Males, however, proved a bit trickier.
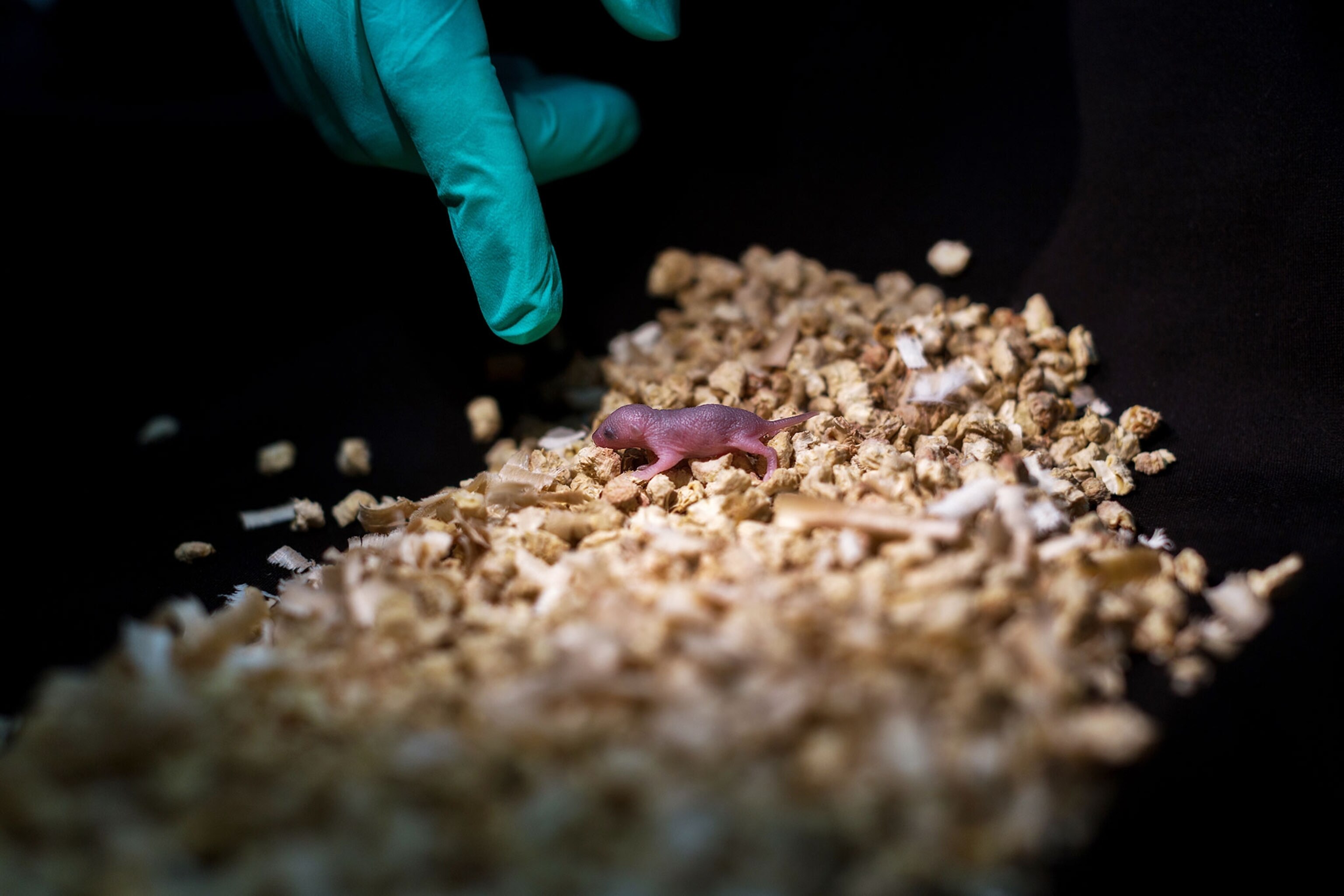
“To make an individual, you have to have an egg; males don't have eggs,” says Richard Behringer, a developmental biologist at the University of Texas who was not involved in the study. So the team instead injected the sperm and the haploid embryonic stem cells into an immature egg stripped of its nucleus, the part of a cell that carries the majority of its genetic material.
At first, the researchers found that if they inserted this modified egg into a uterus, it wouldn't grow. They had to foster its growth outside of the uterus before eventually inserting the developing pups into the surrogate.
This difficulty was somewhat expected. Fathers birthing young without female input is exceedingly rare in nature, notes first author Zhikun Li. “Before we started our work, we didn’t even know whether the bipaternal reproduction could be crossed or not,” he writes in an email.
“A big ethical question mark”
This is not the first time that two female mice bore a live mouse pup. In 2004, researchers announced the birth of the first mice from a maternal duo created using similar gene editing techniques.
“But they took it further,” Monika Ward of the University of Hawai'i, says about the new research. Not only did the Chinese team attempt to refine the method, employing haploid embryonic stem cells, and apply it to pups from males, but they also attempted to dissect the impacts of removing various imprinted regions, Ward says.
For the female pups, the snipping of the third region seemed to allow the pups to grow at a normal rate. For the male pups, deleting the seventh imprinted region allowed the babies to develop to full term and reduced the swelling and breathing issues seen with just six genetic deletions.
Overall, researchers who reviewed the paper praised the rigorousness of the work. “I can't imagine asking them to do more,” Behringer says. It's unclear, however, how this method might be used in the future—and what it might mean for humans.
“It is really a big ethical question mark,” Ward says.
Even though the pups from two moms grew normally for the most part and had their own young, they might still suffer undetected developmental issues and would benefit from a much more detailed health analysis, Surani says. It's also still unknown why the pups from the males died so quickly. Overall, genes from the males required a large amount of manipulation to get the embryos to fully develop, Surani says. There may be lingering imprinted regions that prevented their survival.
“First and foremost to me, in all of these things, is a safety question,” Suter says. “And that's a big hurdle to overcome.”
Of course, even if the method eventually works perfectly in mice, making the leap to humans is no small feat. The similarity in imprinting patterns between mice and humans remains a big question. And many of the current medical tests done in mice are “ethically impossible” in humans, notes one 2011 studyin Genome Biology. The researchers hope to continue honing their methods in other animals, including monkeys.
Opening developmental doors
Even so, the information gleaned from these latest experiments is important and could help us better understand the role of various genes in development.
“If they continue with this, and they play with these imprinted genes ... than we learn a lot about it,” Ward says. Genomic imprinting is thought to play a range of rolesin the development of traits, diseases, and even how well other infertility treatments work.
The research may also serve as yet another call to consider opening up research on embryonic stem cells and gene editing in the U.S., where funding is severely limited for this type of work, and a complex network of laws restricts the breath of studies.
“Because we've had so much limitation on this kind of research, I don't think we really thought it through,” Suter says. What's considered safe? What is adequate justification for its use? Who will get access to such technologies? After all, if the technology develops under the right ethical and medical guidelines, it could give hope for same-sex couples to have genetically related children, imparting similar access to pregnancy assistance that other couples already have.
“I personally think that if we view the inability of opposite sex couples to reproduce as something that deserves technological intervention,” Suter says, “then it seems to me that I don't think we can make a coherent argument against letting same sex couples do the same thing.”
Related Topics
You May Also Like
Go Further
Animals
- Octopuses have a lot of secrets. Can you guess 8 of them?
- Animals
- Feature
Octopuses have a lot of secrets. Can you guess 8 of them? - This biologist and her rescue dog help protect bears in the AndesThis biologist and her rescue dog help protect bears in the Andes
- An octopus invited this writer into her tank—and her secret worldAn octopus invited this writer into her tank—and her secret world
- Peace-loving bonobos are more aggressive than we thoughtPeace-loving bonobos are more aggressive than we thought
Environment
- This ancient society tried to stop El Niño—with child sacrificeThis ancient society tried to stop El Niño—with child sacrifice
- U.S. plans to clean its drinking water. What does that mean?U.S. plans to clean its drinking water. What does that mean?
- Food systems: supporting the triangle of food security, Video Story
- Paid Content
Food systems: supporting the triangle of food security - Will we ever solve the mystery of the Mima mounds?Will we ever solve the mystery of the Mima mounds?
- Are synthetic diamonds really better for the planet?Are synthetic diamonds really better for the planet?
- This year's cherry blossom peak bloom was a warning signThis year's cherry blossom peak bloom was a warning sign
History & Culture
- Strange clues in a Maya temple reveal a fiery political dramaStrange clues in a Maya temple reveal a fiery political drama
- How technology is revealing secrets in these ancient scrollsHow technology is revealing secrets in these ancient scrolls
- Pilgrimages aren’t just spiritual anymore. They’re a workout.Pilgrimages aren’t just spiritual anymore. They’re a workout.
- This ancient society tried to stop El Niño—with child sacrificeThis ancient society tried to stop El Niño—with child sacrifice
- This ancient cure was just revived in a lab. Does it work?This ancient cure was just revived in a lab. Does it work?
- See how ancient Indigenous artists left their markSee how ancient Indigenous artists left their mark
Science
- Jupiter’s volcanic moon Io has been erupting for billions of yearsJupiter’s volcanic moon Io has been erupting for billions of years
- This 80-foot-long sea monster was the killer whale of its timeThis 80-foot-long sea monster was the killer whale of its time
- Every 80 years, this star appears in the sky—and it’s almost timeEvery 80 years, this star appears in the sky—and it’s almost time
- How do you create your own ‘Blue Zone’? Here are 6 tipsHow do you create your own ‘Blue Zone’? Here are 6 tips
- Why outdoor adventure is important for women as they ageWhy outdoor adventure is important for women as they age
Travel
- This royal city lies in the shadow of Kuala LumpurThis royal city lies in the shadow of Kuala Lumpur
- This author tells the story of crypto-trading Mongolian nomadsThis author tells the story of crypto-trading Mongolian nomads
- Slow-roasted meats and fluffy dumplings in the Czech capitalSlow-roasted meats and fluffy dumplings in the Czech capital








